Primary Focal Plantar
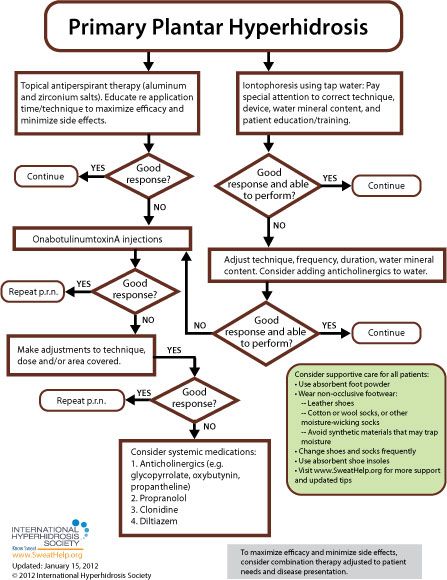
When approaching treatment to primary focal plantar hyperhidrosis, the general recommendation is to try more conservative therapy before resorting to invasive treatment. While each of the anatomic areas prone to excessive sweating is more or less susceptible to the various approaches available, [9] treatment guidelines for primary focal plantar hyperhidrosis are as follows:
An important first step in the care of the plantar hyperhidrosis patient is to provide education regarding good foot hygiene (to keep the feet as dry as possible.) All patients should be advised to wear non-occlusive footwear (made of breathable materials like leather) and moisture-wicking socks (including wool, silk, and "athletic" synthetic fabrics, or cotton socks (but these will need to be changed more often). Any fabrics that trap moisture should be avoided. Patients should also be encouraged to change their shoes and socks frequently and to use absorbent foot powder and absorbent shoe insoles. [145,148] "Shoe driers" are useful for extending the life of footwear, drying them between wearings, and reducing odor. Indeed, patients may be sensitive about the odor caused by plantar hyperhidrosis. They may request recommendations on methods to control odor, especially if they are unable to afford or tolerate treatment. Some suggestions that may help are citric acid products or topical antibiotics, in addition to consistently changing socks to maintain dryness. [160]
For many patients, medical treatment will begin with topical antiperspirants (active ingredient often aluminum chloride hexahydrate). In fact, topical aluminum chloride antiperspirants are often considered the first line of treatment for plantar hyperhidrosis. A high concentration (up to 30%) of active ingredients will, however, probably be required.[145] A two-week trial of topical antiperspirants (with compliance to regimen) is usually sufficient to determine whether topical agents will work for a particular patient. [160]
Topical antiperspirants can be very effective but are limited by irritation that is caused by the formation of hydrochloric acid in a chemical reaction between the aluminum chloride and sweat present on the skin surface. Application on a very dry, nonoccluded skin surface can reduce this irritation substantially. [154]
Generally, it's recommended that prescription antiperspirants containing aluminum chloride be applied before sleep (when sweating is at its most minimal) and washed off 6 to 8 hours later. Patients should be reminded not to put on socks until the product has dried entirely. [160] Skin should be completely sweat-free before product application [157] -- a cool blow drier or towel may be used if necessary. Washing the skin before application is not necessary and may actually lead to greater irritation.
If skin irritation occurs, topical hydrocortisone cream treatment is often recommended. In the absence of skin irritation, the antiperspirant should be applied every night for 1 week until sweating is reduced. Once the antiperspirant has taken effect, the period between treatment re-application may be extended to once per week, or less frequently, as long as desired results are maintained. [155]
Tap water iontophoresis is also considered a first line treatment for plantar sweating. [7,145] Side effects are generally limited to mild stinging and redness. Treatment should be provided three to five times per week until the patient achieves dryness, generally at two to four weeks, and then spaced out to longer intervals to maintain satisfaction. [154] These iontophoresis devices are currently available in the U.S.: the Drionic, The Fischer (by R.A. Fischer) and Hidrex USA's DVP1000. These iontophoresis devices have been cleared by the U.S. Food and Drug Administration to treat hyperhidrosis. With iontophoresis, the patient avoids the irritation often associated with aluminum chloride use and, after training, can perform the procedure at home. [7, 58] The efficacy of iontophoresis depends a lot on correct technique, the device used, adequate mineral content in the water used, and patient training (when performing home treatment). For information about recommended techniques and regimens, click here.
In addition to simple tap water iontophoresis, clinicians have also used iontophoresis to deliver anticholinergics and other drugs to areas affected by hyperhidrosis.[4] This may increase the duration of dryness. [154]
If symptoms do not resolve with topical antiperspirants or iontophoresis, the next option to consider is onabotulinumtoxinA.[95] The U.S. Food & Drug Administration (FDA) has approved onabotulinumtoxinA for the treatment of severe primary axillary hyperhidrosis in patients unable to obtain relief with antiperspirants (July 19, 2004). While only FDA-approved for axillary hyperhidrosis in adults, onabotulinumtoxinA can also be used for any area of focal hyperhidrosis and is commonly used in pediatric patients. [154] OnabotulinumtoxinA injections offer a minimally invasive treatment option and should be repeated as necessary to control symptoms. [99] When treating plantar hyperhidrosis with onabotulinumtoxinA, both physicians and patients should understand that due to the large surface area usually requiring treatment, up to 36 injections may be necessary. And, because of the sensitivity of the feet, the procedure may be "more tedious and uncomfortable" than it would be for palmar or axillary hyperhidrosis. [29] Nerve blocks (posterior tibial and sural nerves) or cyroanesthesia (using ice and pressure) may alleviate some discomfort. [160] But, because of the pain associated with onabotulinumtoxinA injections in the sole, many patients choose iontophoresis as the mainstay of plantar hyperhidrosis treatment over onabotulinumtoxinA. [160]
Transient small muscle weakness occurs occasionally when treating with onabotulinumtoxinA, but compensatory sweating is rare. [154] If the desired results are not achieved initially, practitioners are advised to make adjustments to technique, dose, and/or the area covered and repeat the treatment. Although some patients relapse shortly after their course of treatment, most studies report that a second set of injections will often provide effective symptom abatement. [156]
If the patient does not respond to or does not tolerate onabotulinumtoxinA, another therapeutic option to consider is the use of a systemic medication (glycopyrrolate, oxybutynin, propantheline, propranolol, clonidine, or diltiazem). Additionally, if symptoms are exacerbated in known anxiety-provoking situations, short-term expectant use of a benzodiazepine or anticholinergic may be considered. [9,19]
Oral medications require patient education regarding potential side effects (such as dry mouth, blurred vision, urinary retention, tachycardia and constipation). These side effects may limit the use of anticholinergics in many patients [154, 155] but sometimes can be managed by adjusting the individual's dose. Before treatment with oral systemic medications for hyperhidrosis, extra consideration should be given regarding pediatric patients, people who participate in sports, people who work outdoors and any other patients who may potentially cause themselves injury by becoming overheated. Of interest to practitioners with pediatric patients, the FDA has approved (July 2010) a liquid form of the anticholinergic glycopyrrolate (brand name, Cuvposa) to reduce drooling in pediatric cerebral palsy patients – off-label use of this medication may provide flexibility when dosing for pediatric patients. Watch our expert-led webinar about oral medications to treat hyperhidrosis.
Many practitioners also find that combinations of therapies can be effective. For example antiperspirants to increase duration between onabotulinumtoxinA injections. Adapting and integrating different available options to meet individual patient's needs can improve satisfaction.
Please keep in mind that ETS Surgery is NOT recommended for sweaty feet. NOT even as a last resort.