Primary Focal Palmar
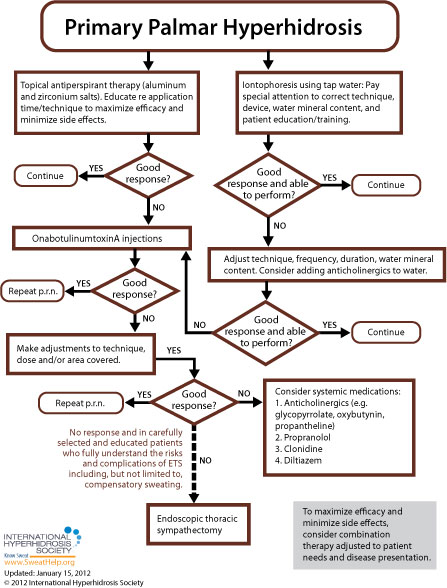
When approaching treatment to primary focal palmar hyperhidrosis, the general recommendation is to try more conservative therapy before resorting to invasive treatment. While each of the anatomic areas prone to excessive sweating is more or less susceptible to the various approaches available, [9] treatment guidelines for primary focal palmar hyperhidrosis are as follows:
For many patients, treatment will begin with topical antiperspirants (active ingredient often aluminum chloride hexahydrate). A higher concentration of active ingredients will often be required for palmar treatment as compared to axillary treatment (up to 30% for palms compared to 20% for the underarms).[145] A two-week trial of topical antiperspirants (with compliance to regimen) is usually sufficient to determine whether topical agents will work for a particular patient. [160]
Topical antiperspirants can be very effective, but are limited by irritation that is caused by the formation of hydrochloric acid in a chemical reaction between the aluminum chloride and sweat present on the skin surface. Application on a very dry, nonoccluded skin surface can reduce this irritation substantially. [154]
Generally, it's recommended that prescription antiperspirants containing aluminum chloride be applied before sleep (when sweating is at its most minimal) and washed off 6 to 8 hours later. Skin should be completely sweat-free before product application [157] -- a cool blow drier or towel may be used, if necessary. Washing the skin before application is not necessary and may actually lead to greater irritation. If skin irritation occurs, topical hydrocortisone cream treatment is often recommended. In the absence of skin irritation, the antiperspirant should be applied every night for 1 week until sweating is reduced. Once the antiperspirant has taken effect, the period between treatment re-application may be extended to once per week, or less frequently, as long as desired results are maintained. [155]
Many experts also consider tap water iontophoresis to be a first line treatment for palmar sweating. [7,145] Side effects are generally limited to mild stinging and redness. Treatment should be provided three to five times per week until the patient achieves dryness, generally at two to four weeks, and then spaced out to longer intervals to maintain satisfaction. [154] These iontophoresis devices are currently available in the U.S.: the Drionic, The Fischer (by R.A. Fischer) and Hidrex USA's DVP1000. These iontophoresis devices have been cleared by the U.S. Food and Drug Administration to treat hyperhidrosis. With iontophoresis, the patient avoids the irritation often associated with aluminum chloride use and, after training, can perform the procedure at home. [7,58] The efficacy of iontophoresis depends a lot on correct technique, the device used, adequate mineral content in the water used, and patient training (when performing home treatment). For information about recommended techniques and regimens, click here.
In addition to simple tap water iontophoresis, clinicians have also used iontophoresis to deliver anticholinergics and other drugs to areas affected by hyperhidrosis.[4] This may increase the duration of dryness. [154]
While antiperspirants or iontophoresis are usually recommended as first line treatments for primary palmar hyperhidrosis, there is a possible exception if the patient's excessive sweating symptoms occur during, or are exacerbated by, known anxiety-provoking situations such as presentations at work, dramatic performances, etc. In these cases, the patient may be treated prior to such events with an anticholinergic or a short course benzodiazepine. [9,19] More information regarding anticholinergic and other oral treatments may be found below.
If symptoms do not resolve with topical antiperspirants, iontophoresis, or short course oral medications (if appropriate), the remaining options to consider are: onabotulinumtoxinA injections, on-going use of systemic medications, Qbrexza topical cloth (used off label), or endoscopic thoracic sympathectomy (ETS).[95] Since systemic medications are often associated with side effects precluding long-term treatment and ETS is invasive and associated with high rates of compensatory hyperhidrosis, onabotulinumtoxinA is recommended first and repeated as necessary to control symptoms.[95]
The U.S. Food & Drug Administration (FDA) has approved onabotulinumtoxinA for the treatment of severe primary axillary hyperhidrosis in patients unable to obtain relief with antiperspirants (July 19, 2004). While only FDA-approved for axillary hyperhidrosis in adults, onabotulinumtoxinA can also be used for any area of focal hyperhidrosis and is commonly used in pediatric patients. [154] OnabotulinumtoxinA injections offer a minimally invasive treatment option and should be repeated as necessary to control symptoms. [99] The average duration of improvement is four to five months for the palms. [154] Generally patients receive two treatments per year. The main side effect is discomfort during injection, which is encountered most often when treating the palms and soles of the feet. Skill and precision is required when treating these areas because of their dense innervation. Adequate anesthesia is necessary for patient comfort. Nerve blocks have been the gold standard of anesthesia for palmar hyperhidrosis. Wrist blocks generally involve the ulnar and median nerves, and sometimes the radial nerve. Nerve blocks allow for more precise injections and better patient comfort because the hand is completely anesthetized. More recently, vibration or cyroanesthesia using ice and pressure has become more prevalent. Cold packs, liquid nitrogen, or ethyl chloride may be used instead of ice.[160] Transient small muscle weakness occurs occasionally when treating the palms with onabotulinumtoxinA, but compensatory sweating is rare. [154] If the desired results are not achieved initially, practitioners are advised to make adjustments to technique, dose, and/or the area covered and repeat the treatment. Although some patients relapse shortly after their course of treatment, most studies report that a second set of injections will often provide effective symptom abatement. [156]
A newer topical treatment dispensed for daily application as a cloth, containing an anticholinergic, and called Qbrexza may have promise for treating sweaty palms. FDA-approved to treat axillary hyperhidrosis, there is potential for Qbrexza's off-label use for palms. IHhS co-founder and board member Dr. David Pariser has led research on the best technique for the use of Qbrexza for palmar Hh. The study compared four different ways of using Qbrexza to manage palm sweating. Results were published in the May 2022 edition of Journal of Drugs in Dermatology. One hundred and twenty patients, age nine years or older with self-reported excessive palm sweating took part in the study, which lasted four weeks and included four different daily palmar Qbrexza regimens. The study found that the application of the Qbrexza cloth onto both hands (until the cloth was dry or about 3 minutes), wearing clean cotton gloves for 30 minutes, and then washing the hands achieved the best results with the most acceptable safety profile. For this group, “hand sweat severity” dropped an average of 4 points out of a 10-point scale. To learn more about this research, read our blog or the full study.
Oral systemic medications including anticholinergics (glycopyrrolate, oxybutynin, and propantheline), propranolol, clonidine, and diltiazem may be used to treat primary palmar hyperhidrosis, but require patient education regarding potential side effects (such as dry mouth, blurred vision, urinary retention, tachycardia and constipation). These side effects may limit the use of anticholinergics in many patients [154, 155] but sometimes can be managed by adjusting the individual's dose. Before treatment with oral systemic medications for hyperhidrosis, extra consideration should be given regarding pediatric patients, people who participate in sports, people who work outdoors and any other patients who may potentially cause themselves injury by becoming overheated. Of interest to practitioners with pediatric patients, the FDA has approved (July 2010) a liquid form of the anticholinergic glycopyrrolate (brand name, Cuvposa) to reduce drooling in pediatric cerebral palsy patients – off-label use of this medication may provide flexibility when dosing for pediatric patients. Watch our expert-led webinar about oral medications to treat hyperhidrosis.
Finally, for carefully selected patients, endoscopic thoracic sympathectomy (ETS) may be an option if all other treatment options have been exhausted (including rounds of treatment regimen adjustments, retreatment, and combination therapies). If ETS is to be pursued, patients must be educated to fully understand the risk of complications including, but not limited to, compensatory sweating. [9] Compensatory sweating is the most common reason for lack of satisfaction with ETS.[72,152]